ISO 80369-7 Luer Connector Gauge with 6% Tape
2026-01-09 18:32:33
hj2008mt
ISO 80369-7:2021 – Dimensional and Performance Standards for Luer Connectors and Reference Gauges
In medical device engineering, small-bore connector integrity is essential for patient safety and system reliability. ISO 80369-7:2021, "Small-bore connectors for liquids and gases in healthcare applications - Part 7: Connectors for intravascular or hypodermic applications," defines stringent dimensional and functional criteria for Luer connectors. This standard replaces ISO 594-1 and ISO 594-2, incorporating improved tolerances, material classifications, and testing protocols to minimize misconnections and leaks in vascular systems.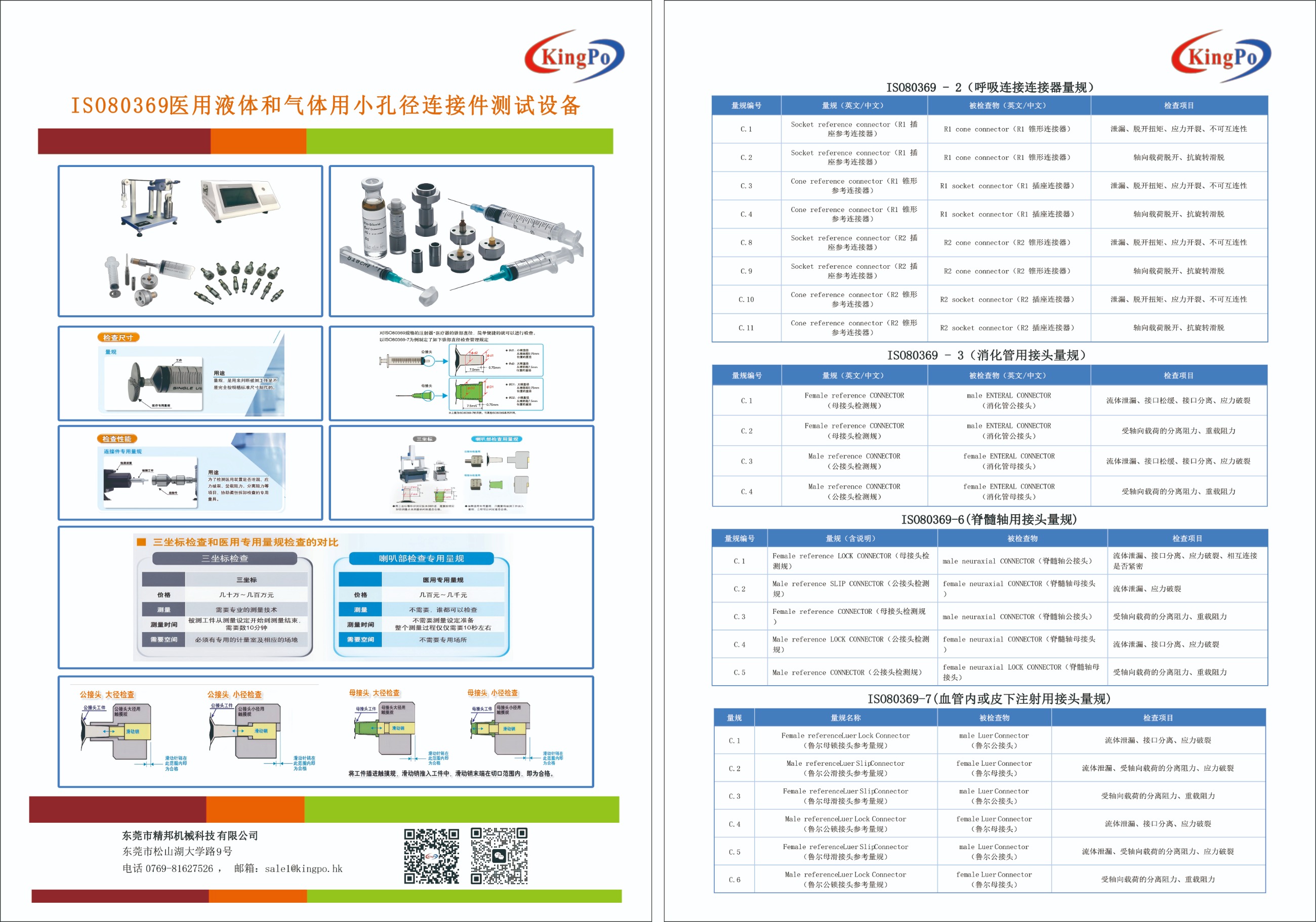 ISO 80369-7 Male Plug Gauge for Luer Connectors
ISO 80369-7 Male Plug Gauge for Luer Connectors
 ISO 80369-7 Male Plug Gauge for Luer Connectors
ISO 80369-7 Male Plug Gauge for Luer ConnectorsThis technical overview examines ISO 80369-7:2021 in depth, emphasizing minimum standards for male reference plug gauges used to verify female Luer connectors. It includes technical specifications, gauge roles in compliance, key features, and quality assurance implications.
Overview of ISO 80369-7:2021 Standard
ISO released ISO 80369-7:2021 in May 2021 for 6% (Luer) taper small-bore connectors in intravascular or hypodermic applications. It covers slip and lock Luer designs, ensuring non-interconnectability with other ISO 80369 series to avoid cross-connections between different medical systems.
Revisions from 2016 include refined tolerances for manufacturability, distinctions between semi-rigid (700-3,433 MPa modulus) and rigid (>3,433 MPa) materials, and enhanced usability assessments. These align with ISO 80369 goals, stressing tests for fluid/air leakage, stress cracking, axial separation resistance, unscrewing torque, and overriding prevention. Male Reference Plug Gauges in Compliance Verification
Male Reference Plug Gauges in Compliance Verification
 Male Reference Plug Gauges in Compliance Verification
Male Reference Plug Gauges in Compliance VerificationMale reference plug gauges serve as "go/no-go" tools to evaluate female Luer connector dimensional accuracy and functional performance. They replicate the standard's conical taper and thread profiles to detect defects that could cause clinical issues.
Gauges assess taper conformity, thread compatibility, and seal efficacy under conditions like 300 kPa pressure. This is vital for intravenous therapy, hypodermic injections, and fluid delivery, where deviations may cause leaks or contamination.
Reputable manufacturers produce gauges from hardened steel (HRC 58-62) with ISO 17025 calibration for traceability. The 6% taper matches the standard's profile for non-interconnectability and performance testing requirements.
Example Product Specifications: Kingpo ISO 80369-7 Male Plug Gauge
| Parameter | Specification |
|---|---|
| Place of Origin | China |
| Brand Name | Kingpo |
| Model Number | ISO 80369-7 |
| Standard | ISO 80369-7 |
| Material | Hardness Steel |
| Hardness | HRC 58-62 |
| Certification | ISO 17025 Calibration Certificate |
| Key Design Features | 6% taper; 300 kPa pressure rating |
Key Specifications and Requirements for Compliant Gauges
ISO 80369-7:2021 specifies reference connectors as gauge benchmarks with the following critical requirements:
Dimensional Tolerances – Annex B drawings for slip and lock connectors ensure leak-proof fits
Material and Hardness – Hardened steel (HRC 58-62) withstands repeated use
Pressure Rating – Validation at 300 kPa simulates medical fluid pressures
Performance Tests (Clause 6) – Comprehensive testing protocols for reliability verification
Mandated Performance Tests
| Test Type | Requirement/Details | Minimum Performance |
|---|---|---|
| Fluid Leakage | Pressure decay or positive pressure method | No leakage |
| Sub-Atmospheric Air Leakage | Vacuum application | No leakage |
| Stress Cracking Resistance | Chemical exposure and load | No cracking |
| Resistance to Axial Separation | Slip: 35 N; Lock: 80 N (minimum hold) | Sustained for 15 s |
| Unscrewing Torque (Lock only) | Minimum torque to resist loosening | ≥ 0.08 N*m |
| Resistance to Overriding | Prevent thread damage during assembly | No overriding |
Enhancing Quality Control and Regulatory Compliance
Using ISO 80369-7 gauges in protocols detects non-conformities early, lowering recall risks and aligning with FDA 21 CFR and EU MDR requirements. Functional testing ensures seals under stress, preventing clinical adverse events.
Key Benefits of Compliance
Risk mitigation against misconnections causing patient harm
Efficiency through traceable calibration processes
Facilitated market access and regulatory approval
Support for innovative material and design development
Frequently Asked Questions
What are ISO 80369-7:2021's primary objectives?
It defines Luer connector dimensions and performance for safe intravascular connections and misconnection prevention.
How do male reference plug gauges verify female Luer connectors?
They evaluate dimensional accuracy, taper engagement, and performance against Annex C references, including leakage and separation testing.
What distinguishes ISO 80369-7 from ISO 594?
ISO 80369-7 adds stricter tolerances, material classes, and integrated slip/lock testing, prioritizing non-interconnectability.
What materials and hardness are required for gauges?
Hardened steel at HRC 58-62 ensures precision and durability for repeated testing.
Why is the 6% taper critical?
It provides conical conformity for secure, leak-resistant fittings in hypodermic and IV systems.
What functional tests does Clause 6 mandate?
Fluid/air leakage, stress cracking, axial resistance (35-80 N), unscrewing torque (≥0.08 N*m), and overriding prevention.
How does ISO 80369-7 handle material rigidities?
It separates semi-rigid and rigid requirements by modulus for design flexibility.
Where to procure compliant reference gauges?
Suppliers like Kingpo, Enersol, and Medi-Luer offer calibrated products meeting standard requirements.
In summary, ISO 80369-7:2021 advances Luer connector standardization, with male reference plug gauges upholding dimensional and performance thresholds. These tools enable superior safety, compliance, and innovation in medical devices.
- ISO 80369-7 Luer Connector Gauge with 6% Tape
- KINGPO will meet you at the 92nd China International Medical Equipment (Autumn) Expo in 2025
- Is defibrillation protection testing done correctly?
- Fatal mistakes in IPX9K waterproof test: nozzle size and water temperature control, the truth you must know
- Neutral Electrode Temperature-rise Tester: Ensuring Safety in Electrosurgery
- ISO 594 is replaced with ISO 80369
- ISO 80369-7:2016 Connectors with 6% (Luer) taper for intravascular or hypodermic applications What is the ISO 80369-7 standard? What happened to ISO 594-1 and ISO 594-2?
- ISO 80369-3 Test Equipment LIst
- Understanding the Importance of Buying a Luer Connection Test Kit
- Medical Device Pressure Validation: Ensuring Accuracy and Reliability



 ISO 80369-7 reference connector and ISO 80369-20 test apparatus
ISO 80369-7 reference connector and ISO 80369-20 test apparatus